Skin Care
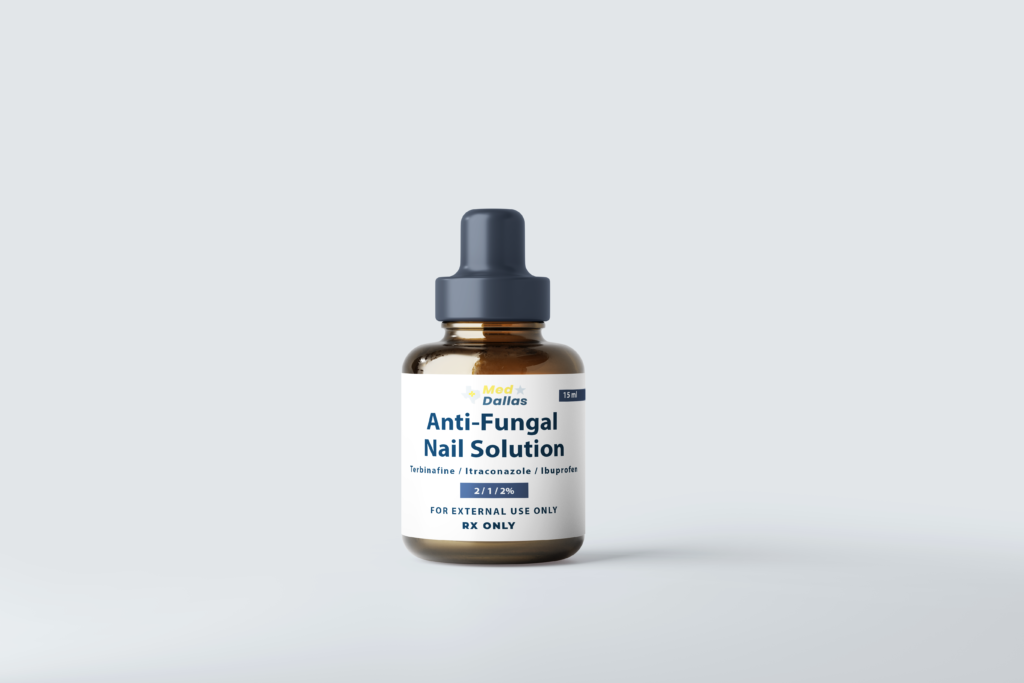
Anti-Fungal Nail Solution
Anti-Fungal Nail Solution (Terbinafine / Itraconazole / Ibuprofen) (15 mL) is a comprehensive treatment formulated to address fungal infections and associated symptoms. This solution combines three powerful ingredients: Terbinafine and Itraconazole, both of which are antifungal agents, and Ibuprofen, an anti-inflammatory drug. Terbinafine and Itraconazole work synergistically to target and eradicate fungal infections such as onychomycosis, while Ibuprofen provides relief from inflammation and discomfort. This solution is designed to offer effective, multi-faceted treatment for both the infection and its symptoms, making it a versatile choice for managing nail fungus and its associated pain.